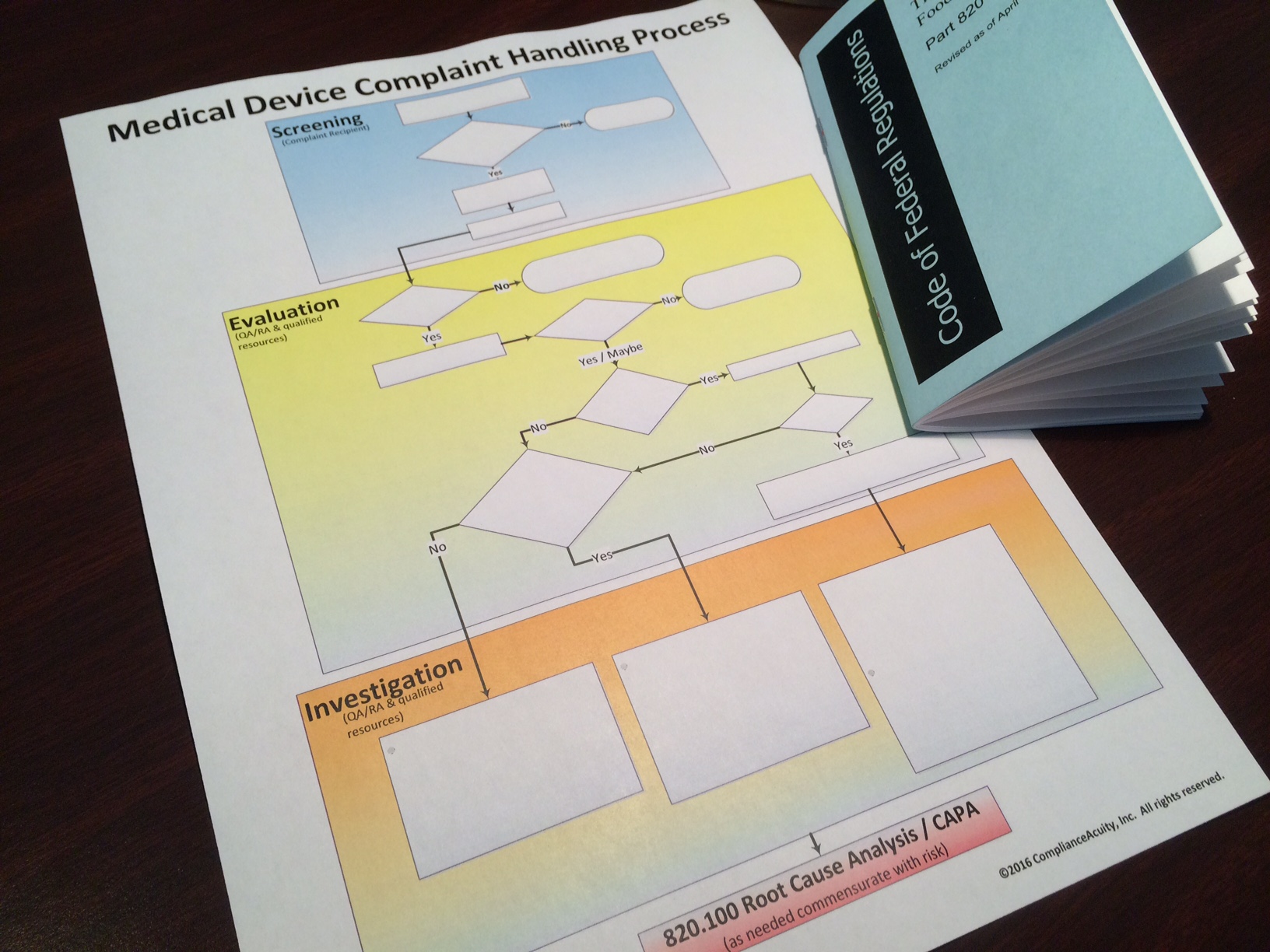
Complaint Handling

Our Industry’s Complaint Handling Challenge:
- In 2015 device quality system FDA-483’s, more Observations (330) were lodged against deficient Complaint Handling procedures than any other subsection of 21 CFR 820 except for CAPA.
- Similarly, in 2015 device quality system Warning Letters, complaint handling (sec. 820.198) citations ranked third (76/690 or 11%) compared to other 21 CFR 820 sections.
- From 2003 to 2007, more than 85% of device quality system Warning Letters cited aspects of the CAPA subsystem (820.100 + 820.198 + 820.90). This has increased to a whopping 92% as of 2015, and is higher than any of the four other major subsystems of 21 CFR 820.
During audits and remediation efforts, ComplianceAcuity has observed that many complaint handling systems are overloaded with misplaced data because:
- They lack an effective mechanism for deciding what feedback statutorily qualifies as a complaint; and
- They lack risk-based tools to consistently gauge severity and decide which complaints require CAPA.
When the wrong data are misdirected into the complaint handling system, it perpetuates customer dissatisfaction and overtaxes resources that are already stressed to begin with. This causes system breakdowns.
The ComplianceAcuity Complaint Handling Solution
When used properly, complaint handling is a powerful process that can strengthen your business by improving customer satisfaction and reducing the cost of quality.
Effective complaint handling is another ComplianceAcuity passion. In fact, the system designed by ComplianceAcuity for a prominent global device manufacturer was featured in by F-D-C Reports in the “The Silver Sheet”. Some of the cornerstones of the ComplianceAcuity complaint handling solution are:
- Standardized pre-screening to ensure that only statutorily-qualified data are put into the system. This prevents the robust complaint handling process from getting overloaded with unnecessary data to begin with.
- Risk-based investigation and improvement actions commensurate with the magnitude of the problem. This optimizes resource allocation and reduces waste.
- Seamless integration with Risk Management and CAPA subsystems to ensure systemic continuity.
- Strategically-deployed failure investigations to efficiently find the real root cause of defects so they go away for good
- Event coding to facilitate data analysis.
Testimonials
“Kevin was very diligent and rigorous in his processing work for the Complaint Handling System. He talked to all those involved to be sure of his facts so that the system was compliant but also reflected the practice, except where we needed to change it…He helped train people where necessary and tried to explain to people why something was needed as opposed to commanding it in place. He communicated with me often enough that I could make management decisions relative to the systems he was working on…It was a pleasure to deal with ComplianceAcuity and would work with them again given the need.”
- Director of Quality, Class II & III Devices