Management Responsibility
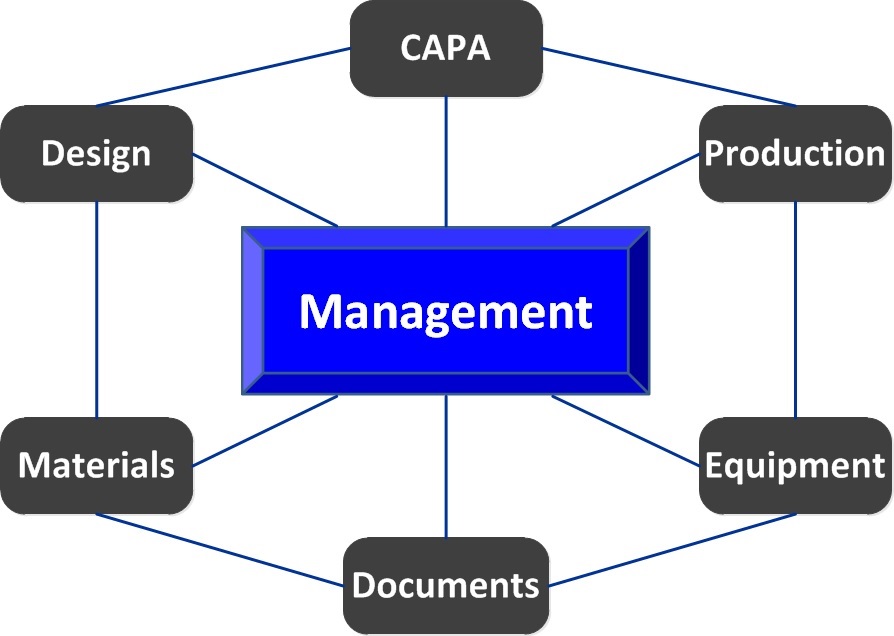
The FDA and international standards bodies recognize that a genuine commitment from executive management is imperative to maintaining a sustaining, functional quality system.
Your highest level of management must establish your company’s quality policy and ensure it is followed (See United States v. Dotterweich, 320 U.S. 277 (1943), and United States v. Park, 421 U.S. 658 (1975).)
ComplianceAcuity can tactfully assist you and your management team in strategically meeting requirements related to:
- Quality Plans and Objectives
- Quality/Regulatory Strategic Planning
- Company-wide management reports
- Comprehensive Management Reviews
- Executive Management Training
Testimonials
“Traditionally our FDA compliance responsibilities have been unclear to us in our unique business model. The explanation that ComplianceAcuityprovided to our management team was the most thorough, tactful, and precise that we’ve ever received. FDA has since concurred with ComplianceAcuity’sanalysis and we continue to rely on ComplianceAcuity’s insight daily as we operate our business.”
- Director of Operations, Class III Devices
